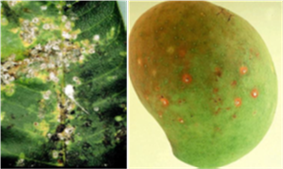
Common Rust in Maize is a major risk for growers in the upcoming season
Last summer season, severe rust infections in crops around the Central Darling Downs were estimated to have caused grain yield losses of 25% or more in some maize corn varieties planted in the late December-early January planting window.
During these events, staff from Black Earth Agronomy forwarded samples of maize corn and sweet corn infested with rust to Dr Sue Thompson, Plant Pathologist with the Centre for Crop Health at USQ. Sue passed the samples on to Roger Shivas at the BRIP Herbarium in Brisbane to confirm the identification of the causal rust species.
All the samples have been identified as common rust, Puccinia sorghi. What the herbarium can't determine is the race of the Puccinia sorghi. However Black Earth staff have observed severe rust infections in varieties of sweet corn in North Queensland which were previously tolerant of the disease. This would indicate a change in the pathogen.
Common rust in corn is caused by the fungus, Puccinia sorghi, which does not infect sorghum as the name would imply.
The optimum temperature for the fungal spores to germinate and infect corn leaves is in the range 15-25 degrees C combined with high humidity or surface moisture for 4 hours or more from dew or rainfall. However the fungus can infect and sporulate (produce secondary spores) from 4-32 degrees C. Consequently the disease is more likely to be a problem in cool, moist conditions which are more likely to occur in the spring and autumn corn crops on the Downs and Lockyer Valley and during the winter months in the warmer areas along the Queensland coast (e.g. Bowen/Burdekin).
Common rust is not a frequent disease problem in maize corn in the main corn belt in the U.S. because resistant or tolerant varieties are grown and because the disease does not survive during the cold winter months. The spores must be carried by the wind from the warmer regions of the USA and Mexico where both maize corn and native corn types persist over winter, before infection can occur. This often happens too late in the corn growing season to have any effect on yield.
My reading of the literature on the net would suggest that common rust is a significant issue in maize corn in Hawaii and parts of South Africa and Argentina where the spores can survive between the seasons on successive plantings of corn or on alternative host plants including creeping oxalis (Oxalis corniculata) or other species of Oxalis which are common broadleaf weeds in gardens and parklands. Yield losses of upto 35% have been reported where severe epidemics have occured in susceptible corn varieties.
This would suggest that we will have to be far more vigilent in scouting maize and sweet corn crops to monitor the levels of rust which may appear if the conditions are favourable. Our experience has shown that the rust can proliferate very rapidly in some varieties of maize corn and sweet corn to the extent that a single application of the fungicide Tilt 250 (propiconazole) at 10 true leaves (V10) could not contain the disease outbreak. Tilt 250 has a permit to control northern leaf blight in maize, sweet corn and popcorn but not common rust. Jerald Pataky from Illinois reported in 2001 that "over a 4 week period, one rust pustule can produce 5,000 spores. Thus, a million spores can be produced on the bottom 3 to four leaves of a corn plant that has as few as 50 to 70 pustules per leaf (about 5% severity)." Apparently Tilt 250 does limit the mycelium growth of rust in the plant leaf tissues but does not kill the spores or stop the spores from germinating on new leaf growth. In practical terms, this means that a spray with propiconazole might be warranted as early as V5 (5 true leaves) if the conditions are favourable and the disease is seen to be moving up the crop canopy.
Given that there would appear to be change in the pathogenicity of common rust to current commercial varieties of maize and sweet corn in Queensland, it would be beneficial if corn and sweet corn growers could obtain Permits for products other than just propiconazole to control the common rust outbreak. Their counterparts in the USA have access to a wide range of Triazoles (e.g. propiconazole, tebuconazole, cyproconazole, epoxiconazole) and Strobilurins (e.g. azoxystrobin, pyrachostrobin) often combined in a single product label mix to improve efficacy and to reduce the likelihood of the development of fungicide resistance.
Products such as azoxystrobin (Amistar) do kill the spores which can significantly slow the rate of development of a rust outbreak, particularly if used in combination with a products from the Triazole family.
Written by
Graham Boulton
Black Earth Agronomy
0429 063907
The information provided above is based on experience and knowledge developed while operating as an Agronomist on the Darling Downs and the Burdekin. The opinions contained within this post are entire that, and may not apply to a grower's specific circumstance. We recommend consulting your own agronomist to ensure best performance on your own farm.